What should be done for efficient and long-lasting lithium-ion batteries?
A passivation layer forms on the negative pole of lithium-ion batteries with some users waiting. Also known as the interface protective film layer (IPF), this layer restricts ion flow, causing an increase in internal resistance inside the battery and, in the worst case, the formation of a lithium coating. It is known to dissolve this layer by charging and cycling more efficiently, and some smartphone users claim they have gained a second or third cycle – albeit a small amount – on their battery.
Scientists do not fully understand the structure of this layer, and some scientists have published on the subject speculating that the cycle of use and performance gain is associated with the disappearance of this passivation layer. Some scientists directly deny the existence of such a layer, saying that this idea is highly speculative and inconsistent with current studies. Whatever the outcome of the lithium-ion passivation debate, there is no parallel between this issue and the "memory" effect of the periodic cycle required to prevent capacity loss in NiCd batteries. The symptoms may seem similar, but the mechanics are different. This effect is also incomparable with sulfation in lead-acid batteries.
A familiar layer deposited on the positive pole is the solid electrolyte interface (SEI). This layer, abbreviated as SEI, actually functions as electrical insulation in a way but lacks the ionic conductivity that allows the battery to perform its functions normally. While the SEI layer reduces capacity, it also protects the battery. Without SEI, Li-ion won't have the longevity it has.
The SEI layer develops as a formation process and battery manufacturers pay great attention to it, as if this is rushed, permanent capacity losses and internal resistance drops can occur in the battery. This process includes multiple cycles, slow charging at elevated temperatures, and rest periods that take weeks to complete. This generation period also aids in battery matching and provides quality control. It also makes it possible to monitor the self-discharge process of the battery by measuring the battery voltage value after rest. Frequent self-discharge of the battery indicates a possible manufacturing defect due to foreign matter contamination.
Electrolyte oxidation (EO) can also occur at the negative pole. This results in a permanent loss of capacity and an increase in internal resistance. There is no way to remove this layer once it has formed, but electrolyte additives can reduce the effects. High temperatures promote electrolyte oxidation while keeping the Li-ion battery at a voltage above 4.10. Field observations show that the combination of temperature and high voltage factors puts more wearisome stress on Li-ion batteries than cycles of abuse.
Lithium-ion is a very clean system that does not need an additional reset after leaving the factory. In addition, it does not need the level of maintenance required in nickel-based batteries. Additional formatting won't do much because the battery is at maximum capacity from the start. (Except for small capacity increases gained during long cooldowns). Once the battery is weak, a full discharge will not improve capacity – low capacity indicates that the battery is nearing the end of its useful life. The charge/discharge process helps to calibrate a “smart” battery but does not improve the chemistry of the battery. Instructions for use recommending charging a new Li-ion battery for 8 hours is an "outdated" habit from the era of old nickel batteries.
What should be done for non-rechargeable Lithium batteries to be efficient and long-lasting?
Basic lithium batteries such as lithium-thionyl chloride (LTC) benefit from the passivation process they spend waiting. The passivation electrolyte is a thin layer formed as a result of the reaction between the lithium-positive pole and the carbon-based negative pole. (Contrary to Li-ion batteries, in lithium batteries, the positive pole is lithium, and the negative pole is from graphite material.)
Without this layer, most lithium batteries cannot function, as lithium leads to rapid self-discharge and wear of the battery. Scientists working on batteries even say that if the lithium chloride layer does not form, the battery may explode, and in this way, the passivation layer ensures the existence of the battery and makes it possible to store it for 10 years.
Temperature and charging conditions can contribute to the formation of the passivation layer. A fully charged Lithium-thionyl chloride (LTC) battery is more difficult to reactivate after a long standby time than a Lithium-thionyl chloride (LTC) battery kept at a low charge. While lithium-thionyl chloride (LTC) batteries should be kept at low temperatures, a temperature increase would be more appropriate to remove the battery from its passivation state, as it will support thermal conductivity and support ions to move more freely in the process.
CAUTION: Do not subject batteries to physical stress or extreme heat. Explosions that occurred as a result of careless handling caused many serious injuries to workers.
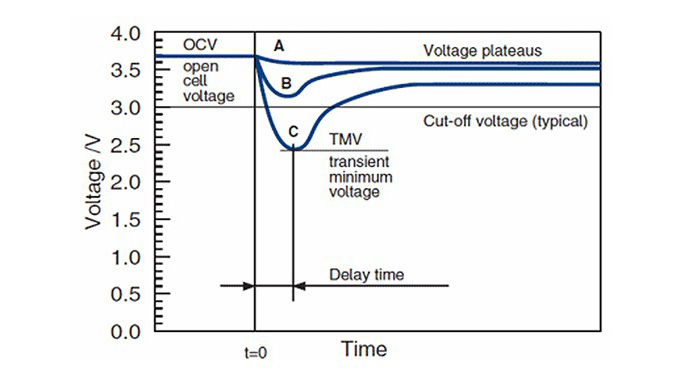
When a load is applied to the battery for the first time, the passivation layer transmits the voltage with a delay and Picture 2 shows the drop and power gain in batteries affected by different passivation levels. Battery A will experience a minimal voltage drop, while the C battery will need some time to recover and regain power.
Picture 2: How does the battery in a passivation state behave when a load is applied?
A battery has a soft passivation level, B needs time to recover, and C is the most affected battery. Research: EE Times
In devices where LTC batteries are used, like a toll sensor, they draw very low currents and can develop a passivation layer that causes dysfunction. The temperature factor also contributes to this development.
A solution to this situation can be found with a large capacitor to be added in parallel with the battery. Having developed a high internal resistance, the battery can continue to charge the capacitor, emitting occasional high waves; The waiting time in between is spent recharging the capacitor.
To help prevent sulfation that can occur when storing batteries, some lithium batteries are shipped with a 36kΩ resistor. This resistor is a charge that clings to the battery, in a way, like a parasite. Constant low discharge current prevents this layer from thickening, but on the other hand, this will shorten the shelf life of the battery. After 2 years of storage with a 36kΩ resistor, it can be said that the batteries still have 90 percent capacity. Another solution is to attach a device that periodically sends discharge waves during storage to the battery.
Not all lithium batteries wake up when placed in a device and a load is applied. The current may be too insufficient to reverse the passivation effect. Also, the device itself may detect and reject the passive battery as very low charge or broken. Most batteries of this type can be prepared by applying a controlled load with a battery analyzer (Cadex). The battery analyzer thus verifies that the battery is functioning properly before it encounters it in the field.
The discharge current required for passivation is C-class between 1C and 3C (up to 3 times the stated capacity). The battery voltage should return to 3.2V when the load is applied; this process typically takes up to 20 seconds. The same process can be repeated, but it should not take more than 5 minutes. The voltage of a properly functioning battery with a 1C load should stay above 3.0V. A drop below 2.7V means the battery is nearing the end of its life.
These lithium-metal batteries are rich in lithium content and require stricter handling rules and stricter precautions when shipping them than Li-ion batteries of the same amperage. Due to the intense energy it contains, it is a must to show high sensitivity while carrying it.
Cautions on the use of lithium batteries.
CAUTION: When charging SLA batteries at high voltage, current must be applied to the location to protect the battery. During charging, the battery voltage and temperature should always be monitored by pulling the current limit to the lowest setting.
In case of rupture of the battery, leakage of electrolyte, or any other case of contact with the electrolyte, immediately pour water on it. If eye exposure occurs, flush your eyes with water for about 15 minutes and consult a physician immediately.
Wear approved goggles in case of contact with electrolyte, lead, or cadmium.
In case of skin contact, wash immediately with water.